We use cutting-edge technologies to deliver comprehensive, innovative, and externally recognized science
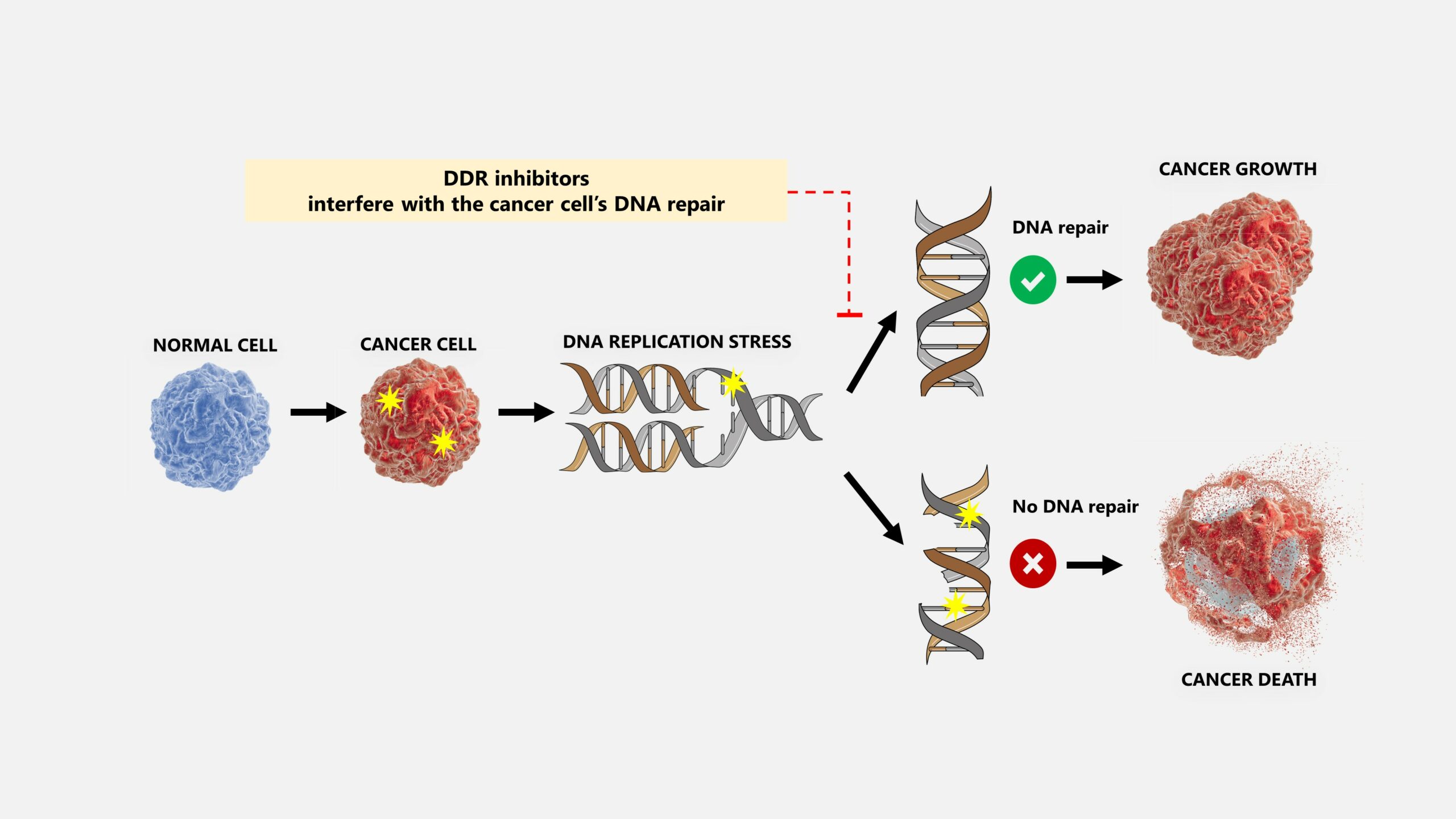
Targeting the DDR
The loss of growth control mechanisms in cancer cells results in abnormal growth and damage to macromolecules such as DNA. Defects in the DNA repair and DNA damage response (DDR) in cancer cells give rise to genomic instability, aiding cancer progression via mutation accumulation. However, it also provides targetable vulnerabilities relatively specific to cancer cells that can be exploited for clinical benefit. Our strategy targets these mechanisms in a novel way so that cancer cells accumulate excessive amounts of DNA damage that they can no longer repair, ultimately leading to their death. This works similarly to chemo- and radiotherapy, which also causes DNA damage to kill cancer cells. The difference is that chemo- and radiotherapy also causes damage to normal cells, resulting in severe side effects. As normal cells have fewer DNA lesions than cancer, inhibiting DNA repair will specifically target the cancer cells while sparing the normal cells and not generate serious adverse effects.
Multilevel crosstalk between metabolism and DDR consists of the regulation of nucleotide supply critical for DNA repair and the direct involvement of metabolic enzymes in the repair processes. The one-carbon metabolism pathway transfers and incorporates carbon groups obtained from the diet to produce purine and pyrimidine nucleotides, which are essential for DNA synthesis and repair. Cancer cells rely heavily on this pathway for nucleotide supply and general regulation of nucleotide pools. Cancer cells are more sensitive to nucleotide pool imbalances than normal cells, and such imbalances also lead to DNA damage.
At One-carbon therapeutics, we take advantage of our deep biological understanding of metabolism and DNA repair processes to develop novel therapeutic approaches targeting both. This is resulting in more efficient, cancer-specific drugs and novel synthetic lethal combination approaches.
Supporting fast growth through DNA building blocks
Many one-carbon metabolizing enzymes, including methylenetetrahydrofolate dehydrogenase 1 and 2 (MTHFD1/2), are associated with the development of cancers. MTHFD2 is an oncofetal protein; under normal circumstances, it does not exist in healthy adult tissue; it is present only in embryos before cells mature into specialized organs. It is re-expressed in cancers and associated with poor disease outcomes. While not oncofetal, MTHFD1 is also associated with malignancy, and depletion of both MTHFD1 and 2 proteins abrogates malignant phenotypes, such as proliferation, migration, invasion, and metastasis in various cancer models. More recent research shows that both MTHFD1 and 2 have additional non-enzymatic and metabolism-related functions that are important for epigenetic and DDR processes in cancer cells. Since the expression and functions of MTHFD1/2 proteins are cancer-specific, our MTHFD1/2 inhibitor is causing replication stress and death in cancer cells while sparing normal cells.
Menu bar
